Organelles Found in Bacteria
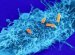
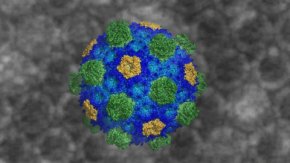 Researchers at Berkeley Lab and MSU have obtained the first atomic-level view of an intact bacterial microcompartment, shown here. Credit: Markus Sutter/Berkeley Lab and MSU
Researchers at Berkeley Lab and MSU have obtained the first atomic-level view of an intact bacterial microcompartment, shown here. Credit: Markus Sutter/Berkeley Lab and MSU
Scientists are providing the clearest view yet of an intact bacterial microcompartment, revealing at atomic-level resolution the structure and assembly of the organelle's protein shell.
The work, led by scientists at the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) and Michigan State University (MSU), will appear in the June 23 issue of the journal Science. They studied the organelle shell of an ocean-dwelling slime bacteria called Haliangium ochraceum.
"It's pretty photogenic, " said corresponding author Cheryl Kerfeld, a Berkeley Lab structural biologist with a joint appointment as a professor at the MSU-DOE Plant Research Laboratory. "But more importantly, it provides the very first picture of the shell of an intact bacterial organelle membrane. Having the full structural view of the bacterial organelle membrane can help provide important information in fighting pathogens or bioengineering bacterial organelles for beneficial purposes."
 These organelles, or bacterial microcompartments (BMCs), are used by some bacteria to fix carbon dioxide, Kerfeld noted. Understanding how the microcompartment membrane is assembled, as well as how it lets some compounds pass through while impeding others, could contribute to research in enhancing carbon fixation and, more broadly, bioenergy. This class of organelles also helps many types of pathogenic bacteria metabolize compounds that are not available to normal, non-pathogenic microbes, giving the pathogens a competitive advantage.
These organelles, or bacterial microcompartments (BMCs), are used by some bacteria to fix carbon dioxide, Kerfeld noted. Understanding how the microcompartment membrane is assembled, as well as how it lets some compounds pass through while impeding others, could contribute to research in enhancing carbon fixation and, more broadly, bioenergy. This class of organelles also helps many types of pathogenic bacteria metabolize compounds that are not available to normal, non-pathogenic microbes, giving the pathogens a competitive advantage.
The contents within these organelles determine their specific function, but the overall architecture of the protein membranes of BMCs are fundamentally the same, the authors noted. The microcompartment shell provides a selectively permeable barrier which separates the reactions in its interior from the rest of the cell. This enables higher efficiency of multi-step reactions, prevents undesired interference, and confines toxic compounds that may be generated by the encapsulated reactions.
Cheryl Kerfeld and Markus Sutter handle crystallized proteins at Berkeley Lab's Advanced Light Source. Credit: Marilyn Chung/Berkeley Lab